Helpful Articles
From Our Team

SORT BY TOPIC

Patagonia Health News
Patagonia Health Celebrates the Success of User Group Events
READ MORE >

Practice Management,
Healthcare Technology
Best Features for a Practice Management Software
READ MORE >

Behavioral Health,
Healthcare Technology
What to Look for in Substance Abuse Software for Your Treatment Center
READ MORE >

Data Security,
Expert Interviews
Expert Interview: Brian Scalia | Aligning System Updates with Healthcare Regulations and User Needs
READ MORE >

Behavioral Health,
Data Security
Choosing a HIPAA-Compliant Mental Health EHR
READ MORE >

Behavioral Health,
Healthcare Technology
How EHR Systems Streamline Mental Health Assessments
READ MORE >

Behavioral Health,
Medical Billing
Finding the Right Billing Software for Therapists
READ MORE >

Behavioral Health,
Healthcare Technology
How EHRs Empower Trauma-Informed Care
READ MORE >
Public Health,
Patient Experience
How an EHR Improves Patient Safety
READ MORE >

Behavioral Health,
Medical Billing
How Accounting and Billing Technology Empowers Therapists
READ MORE >

Behavioral Health,
Interoperability
How to Improve Interoperability in Behavioral Healthcare
READ MORE >

Behavioral Health
Mastering Clinical Documentation: A Practical Guide to Progress Notes
READ MORE >

Behavioral Health
The Best EHR for Psychiatrists
READ MORE >

Patagonia Health News
Patagonia Health Wins 2025 Stevie Award
READ MORE >

Behavioral Health,
Healthcare Technology
The Ultimate Guide to EHR Implementation for Mental Health Clinics
READ MORE >

Data Security,
Expert Interviews
Expert Interview: Harbi Dhanjal | Securing Your Health Organization
READ MORE >

Patagonia Health News
Patagonia Health Wins Triangle Business Fast 50 Award 2024
READ MORE >

Financial Wellness
Is the Sun Setting on Your Current EHR System?
READ MORE >

Patagonia Health News
Enhanced Custom Forms App by Patagonia Health
READ MORE >

Patagonia Health News
Introducing Patagonia Health's Innovative eLearning Module (eLM)
READ MORE >

Behavioral Health
Breaking the Cycle: The Power of Deflection and Diversion in Preventing Opioid Overdose
READ MORE >

Behavioral Health,
Practice Management
Weaving the Golden Thread for Clinical Documentation
READ MORE >

Healthcare Technology,
Expert Interviews
Expert Interview: Jolie Rollins & Monique Dever
READ MORE >

Industry News,
Interoperability
HHS Proposed HTI-2 Rule to Improve Interoperability
READ MORE >

Behavioral Health,
Industry News
New ASAM Criteria for Behavioral Health Clinicians
READ MORE >

Behavioral Health
Tools for Mobile Integrated Health Services in Behavioral Health
READ MORE >

Behavioral Health
Benefits of Mobile Integrated Health for Behavioral Health Organizations
READ MORE >

Patagonia Health News
Patagonia Health Wins Gold Award for Sales and Customer Service
READ MORE >

Patagonia Health News
Patagonia Health Launches Ideas Portal for Feature Requests
READ MORE >

Medical Billing,
Public Health
They’re Going Around: Local Health Department Billing Headaches
READ MORE >

Medical Billing,
Financial Wellness
Improve Your Collections Process with 5 Easy Changes
READ MORE >

Patagonia Health News
Patagonia Health Promotes Prasad Naik as VP of Finance
READ MORE >

Patagonia Health News
Patagonia Health Celebrates 15 Years of Service
READ MORE >

Patagonia Health News
Patagonia Health Streamlines its Clinical Form Update Process
READ MORE >

Patagonia Health News
Patagonia Health Offers FPAR 2.0 Reporting
READ MORE >

Financial Wellness
Sustainable Healthcare: The Benefits of Going Paperless
READ MORE >

Interoperability,
Financial Wellness
Why Should I Choose a Cures Act Certified EHR?
READ MORE >

6 Strategies to Boost Patient Engagement
READ MORE >

Interoperability,
Financial Wellness
School Health: Why Should My EHR Be FERPA Certified?
READ MORE >

Patient Experience,
Healthcare Technology
How Your EHR Can Improve Health Equity
READ MORE >

Financial Wellness
Managing Your EHR Implementation Timeline
READ MORE >

Public Health
The 5 A’s for Smoking Cessation Interventions
READ MORE >

Financial Wellness,
Healthcare Technology
EHRs Can Improve Efficiency of High Volume Immunizations
READ MORE >

Patagonia Health News
Introducing our New Appointment Adherence App
READ MORE >

Patagonia Health News
“Patagonia Health Pioneers” Recognized for 10+ Years of Service
READ MORE >

Patagonia Health News
Patagonia Health Certified as an NC Minority-Owned Business
READ MORE >

Patagonia Health News
Patagonia Health Wins 2023 Stevie Award
READ MORE >

Public Health,
Healthcare Technology
How EHRs Help Public Health Win the War Against TB
READ MORE >

Financial Wellness
10 Questions to Ask in Your EHR RFP
READ MORE >

Interoperability,
Expert Interviews
Expert Interview: Komal Sadani
READ MORE >

Industry News,
Financial Wellness
The Mental Health Matters Act– EHR Functionalities for School Health
READ MORE >

Patagonia Health News
Patagonia Health Joins Forces with Fairfax County Health Department
READ MORE >

Patagonia Health News
Patagonia Health Wins Triangle Business Fast 50 Award
READ MORE >

Financial Wellness
EHR Selection and Implementation Success: Common Q & As
READ MORE >

Patient Experience,
Patient Portal
How an EHR Improves Patient Care
READ MORE >

Healthcare Technology,
Expert Interviews
Expert Interview: Don Sargent
READ MORE >

Financial Wellness
Your Modern-day EHR Can't Be DIY
READ MORE >

Behavioral Health,
Public Health
Fentanyl Crisis - How an EHR Can Help
READ MORE >

Patient Experience,
Telehealth
Providing a Better Telehealth Experience
READ MORE >

Behavioral Health,
Public Health
Community Violence: How an EHR can Help
READ MORE >
Financial Wellness,
Healthcare Technology
How EHRs Reduce Medical Costs for Providers
READ MORE >

Public Health,
Healthcare Technology
How an EHR Can Support Men’s Health
READ MORE >

Behavioral Health,
Telehealth
Telehealth Group Therapy Functionality Now Expanded
READ MORE >

Patagonia Health News
Patagonia Health Connects to Multiple State-Run Immunization Registries
READ MORE >

Telehealth,
Financial Wellness
Inflation and the Need for Telehealth Access
READ MORE >

Financial Wellness
EHR Total Cost of Ownership: Pricing You can Trust
READ MORE >

Patagonia Health News
Patagonia Health Wins 2022 Stevie Award!
READ MORE >

Patagonia Health News
Patagonia Health Offers Free 2-Factor Authentication for EHR
READ MORE >

Healthcare Technology
Are EHR and EMR the Same Thing?
READ MORE >

Interoperability,
Healthcare Technology
Public Health 3.0 Framework and EHRs to Support it
READ MORE >

Healthcare Technology
EHR Apps Study Spells the Future of Healthcare
READ MORE >

Public Health
Opioid Abuse Prevention in Chronic Pain Patients
READ MORE >

Behavioral Health
Behavioral Health Trends for 2022
READ MORE >

Behavioral Health,
Public Health
Adolescent Mental Health Tips for Caregivers
READ MORE >

Financial Wellness
Tips to Support Employee Mental Health
READ MORE >

Financial Wellness
Employee Retention Strategies in Healthcare
READ MORE >

Industry News
Major Healthcare Regulations for 2022
READ MORE >

Financial Wellness
Personalization, Configuration and Customization in EHR Design
READ MORE >

Financial Wellness
How to Migrate data to a new EHR Successfully
READ MORE >

Financial Wellness
How to Train New Employees in your EHR when you‘re Busy
READ MORE >

Public Health
How EHRs support Diabetes & Smoking Cessation Programs
READ MORE >

Practice Management
What you need to know about FPAR 2.0 for 2022
READ MORE >

Financial Wellness
Implementation Best Practices for EHRs
READ MORE >

Financial Wellness
Cloud Based Complete EHR Systems: Solving the Build it or Buy it Debate
READ MORE >

Patagonia Health News
Jonathan Strange Hired as Patagonia Health's Clinical Director
READ MORE >

Financial Wellness
How to Tell when it’s Time For a New EHR
READ MORE >

Behavioral Health
Adolescent Behavioral Health: Solutions for a National Emergency
READ MORE >

Behavioral Health,
Public Health
Behavioral Health Integration: EHR Support for Co-Treatment
READ MORE >

Patient Experience
Social Determinants of Health: Two Perspectives
READ MORE >

Interoperability,
Practice Management
EHR Interoperability: Playing Well with Others
READ MORE >

Telehealth
Telehealth or Virtual Meetings? How to tell the difference
READ MORE >

Patient Experience,
Healthcare Technology
Whole Person Care and the Role of EHRs
READ MORE >

Patient Experience,
Practice Management
Texting is Now a Vital Part of Patient Communications
READ MORE >

Behavioral Health
IM+Cans: A Paradigm Shift in Behavioral Health Clinical Tools
READ MORE >

Interoperability,
Practice Management
The Cures Act: How EHRs Play a Part
READ MORE >

Industry News,
Interoperability
Trend of Health Information Exchanges Merging and Linking
READ MORE >

Patagonia Health News
Patagonia Health Launches Time and Effort Tracking App
READ MORE >

Data Security
How to Defend Against the Rise of Ransomware
READ MORE >

Financial Wellness
Finding a Trustworthy EHR Vendor
READ MORE >

Patagonia Health News
Patagonia Health Joins the Carequality Network
READ MORE >

Behavioral Health,
Telehealth
Guidelines for Using Telehealth for Group Therapy
READ MORE >

Financial Wellness,
Patient Portal
Alleviate Staffing Shortages with an EHR and Patient Self Services
READ MORE >

Patagonia Health News
Patagonia Health Hires Clark McKenna as New Strategic Account Executive
READ MORE >

Financial Wellness
EHR Implementation Success: 7 Tips
READ MORE >

Data Security
Staff Moves: Increasing Security and Protecting Patient Data
READ MORE >

Patagonia Health News
Customer Service Success: Patagonia Health Wins 2021 Stevie Award
READ MORE >

Telehealth
Benefits of an Embedded EHR Telehealth Tool
READ MORE >

Patagonia Health News
Patagonia Health now supporting MS Edge Browser
READ MORE >

Financial Wellness,
Healthcare Technology
Use a Cloud-Based EHR to Reduce Costs
READ MORE >

Financial Wellness
How to Evaluate EHR Solutions
READ MORE >
Public Health
Modernize Public Health Infrastructure to Defend Against Emerging Viruses
READ MORE >

Patagonia Health News
Patagonia Health Piloting New Contactless Patient Experience App
READ MORE >

Financial Wellness
Usability versus Learnability in EHR User Interface
READ MORE >

Financial Wellness
Shopping for an EHR - 8 things to find out First
READ MORE >

Industry News
Do Individuals have to Disclose Vaccination Status?
READ MORE >

Behavioral Health
EHR Implementation for Behavioral Health Challenges
READ MORE >

Data Security
Protect Patient Data from CyberAttack: 5 EHR Security Features
READ MORE >

Financial Wellness
Combat Digital Burnout with these 3 EHR Solutions
READ MORE >

Healthcare Technology
EHRs for E-Prescriptions and Drug Monitoring Programs
READ MORE >

Medical Billing,
Financial Wellness
Bill to Adopt the CCBHC Medicaid Demonstration Nationwide
READ MORE >

Financial Wellness
Optimize IT Spending: How EHR Support Affects your Bottom Line
READ MORE >

Financial Wellness
Traumatic Stress: Protecting Health Staff
READ MORE >

Industry News,
Data Security
Internet Explorer Security is going away: Is it taking yours with it?
READ MORE >
Public Health
Communication Tips for the Importance of Immunizations
READ MORE >

Patagonia Health News
Patagonia Health Offers Interface to PHIN Public Health Information Network
READ MORE >

Public Health,
Industry News
The Threat to Mental Health during COVID-19 & How Practitioners Can Help
READ MORE >

Patagonia Health News
Patagonia Health: Adapt & Innovate, At Speed
READ MORE >

Patagonia Health News
Patagonia Health Provides Tools to Public Health Departments for Successful Mass Vaccination Initiatives
READ MORE >

Behavioral Health
The Golden Thread: Tell a Story of the Entire Treatment Journey
READ MORE >

Patagonia Health News
Patagonia Health EHR Wins BRONZE Stevie® Award 2020: Customer-Focused Service
READ MORE >
Patagonia Health News
Patagonia Health Develops Integrated Telehealth Solution
READ MORE >

Telehealth
Telehealth is Here to Stay
READ MORE >

Behavioral Health,
Public Health
Telehealth Best Practices: Enhancing "Webside" Manner
READ MORE >

Behavioral Health
Mental Health Awareness Month
READ MORE >

Public Health,
Practice Management
Contact Tracing and Next Steps
READ MORE >
Patagonia Health News
Patagonia Health Signs Contract with Carequality Interoperability Framework
READ MORE >

Public Health,
Patagonia Health News
Patagonia Health Featured in KLAS COVID-19 Technology Guide
READ MORE >

Telehealth,
Industry News
New Rules for Telehealth Technology
READ MORE >

Patagonia Health News
Patagonia Health Releases COVID-19 Risk Assessment and Public Health Management Decision Making Tool
READ MORE >

Financial Wellness
Becoming a Certified Community Behavioral Health Clinic (CCBHC): Part 2
READ MORE >

Behavioral Health
Becoming a Certified Community Behavioral Health Clinic (CCBHC): Part 1
READ MORE >

Behavioral Health,
Interoperability
Why Health Information Exchange is Important for EHR Use
READ MORE >

Interoperability
What is HL7?
READ MORE >

Behavioral Health
Inside Behavioral Health: What is a CCBHC?
READ MORE >

Interoperability
What is Interoperability?
READ MORE >

Industry News
North Carolina Requires Opioid E-Prescribe
READ MORE >

Medical Billing,
Financial Wellness
Connecting to NC HealthConnex
READ MORE >

Financial Wellness,
Healthcare Technology
Ten Indicators That You Should Replace and Modernize Your EHR System
READ MORE >

Interoperability
What is the NC HIE?
READ MORE >

Financial Wellness
Will your staff use and adopt your new Electronic Health Record EHR software?
READ MORE >

Patagonia Health News
Patagonia Health Welcomes Harbi Dhanjal as VP of Engineering
READ MORE >

Patagonia Health News,
Customer Story
Mental Health Association in Passaic County Partners with Patagonia Health
READ MORE >

Patagonia Health News
Patagonia Health Wins 2019 Customer Service Award
READ MORE >

Financial Wellness
6 Ways to Optimize Your EHR
READ MORE >

Behavioral Health,
Interoperability
CMS and ONC Propose New Rules for Interoperability
READ MORE >

Patagonia Health News
Site Code Filter Added to Billing System
READ MORE >

Digital Voice Assistants in Healthcare
READ MORE >

Financial Wellness
Best-of-Breed or All-in-One? Which EHR is Right for You?
READ MORE >

Financial Wellness
3 Important - Often Unasked - Questions to Consider when Selecting a new EHR
READ MORE >
Patagonia Health News
Patagonia Health Expands EHR for Home Visit Intervention Tracking
READ MORE >

Patagonia Health News
Vaccine Inventory App is Enhanced
READ MORE >

Patagonia Health News
Custom Reports Added to EHR
READ MORE >

Patagonia Health News
Patient Consent Forms Are Now Editable
READ MORE >

Patagonia Health News
Patagonia Health Hires Amos Slaymaker as VP of Sales
READ MORE >
Patagonia Health News
Bi-directional Lab Orders Interface
READ MORE >

Patagonia Health News
Behavioral Health Agencies Successfully Connected to NCHIE via Patagonia Health EHR
READ MORE >
Patagonia Health News
Patagonia Health's EHR Software Earns ONC Health IT Certification
READ MORE >

Patagonia Health News
Meaningful Use Stage 3 Certification is Complete!
READ MORE >

Behavioral Health
Having the Right Tool at the Right Time
READ MORE >

Financial Wellness
Not all EHR Vendors are Created Equal
READ MORE >

Behavioral Health,
Public Health
Setting the Stage: Patient Satisfaction Begins at Check-In
READ MORE >

Financial Wellness
Pyramid Power: EHR Support and On-going Training Set Apart the Best from the Rest
READ MORE >

Patagonia Health News
Patagonia Health to Roll out New Patient ID Scanner
READ MORE >

Public Health
The Effects and Hazards of E-cigarettes (Guest Blog by Rebecca Williams, MHS, PhD)
READ MORE >

Patagonia Health News
Patagonia Health Makes Top 10 Most Trusted EHR Vendors List
READ MORE >

Behavioral Health
EHR Use Case for Smoking Behavior Cessation
READ MORE >

Patagonia Health News
Patagonia Health Wins GOLD!
READ MORE >

Public Health
When battling Flu season, EHR technology can help!
READ MORE >

Financial Wellness
Switching from Legacy to Cloud Based EHR
READ MORE >

Patagonia Health News
Patagonia Health EHR Wins GOLD Stevie® Award 2018 Customer Service Success
READ MORE >
Patagonia Health News
Patagonia Health adds New eCQMs to EHR
READ MORE >

Financial Wellness
Will Mental Health Providers get EHR Incentives?
READ MORE >
Healthcare Technology
3 Ways (+1 bonus) EHR Data Helps Calm the Opioid Crisis
READ MORE >

Financial Wellness
In the Aftermath of Hurricanes, Your Choice of EHR Matters
READ MORE >

Patagonia Health News
Patagonia Health Recognized by Healthcare Tech Outlook Magazine as a Top 10 Most Promising EHR Solution for 2017
READ MORE >

Financial Wellness
Is Your EHR Vendor Legit? The Top 4 things to Find Out
READ MORE >

Public Health,
Interoperability
What is an Immunization Registry and How Does your Healthcare Organization Benefit?
READ MORE >

Data Security
2-Factor Authentication: A shield for PHI against hackers
READ MORE >

Patagonia Health News
Save Paper and Time with Patagonia Health’s built-in Electronic Patient Consent Forms
READ MORE >

Behavioral Health
Pushing Mental Healthcare Quality to the Forefront
READ MORE >

Patagonia Health News
Patagonia Health Supports 2-Factor Authentication
READ MORE >
Public Health
User-Centered Design Process
READ MORE >

Public Health
Vaccine-Preventable Diseases: Public Health vs Personal Choice. How can meaningful use EHR help keep public safe?
READ MORE >

Public Health,
Patient Experience
Keeping Patient Safety a Priority in your Health Center
READ MORE >
Behavioral Health,
Public Health
Health in All Policies – What it means for Mental Health
READ MORE >

Financial Wellness
The 6 Steps EHR Selection & Implementation: Plan for Planning
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Julia Caplan, MPP, MPH
READ MORE >

Patagonia Health News
Patagonia Health wins Bronze Stevie® Award in the “ Customer Service Success - Technology Industries” for second year
READ MORE >

Data Security
4 Reasons Not Conducting a Security Risk Assessment Can Cost You Money
READ MORE >

Patagonia Health News
Patagonia Health Partners Offers Security Risk Assessment via databrackets
READ MORE >

Public Health
A Balancing Act for Public Health Departments. Here are three things you should focus on.
READ MORE >
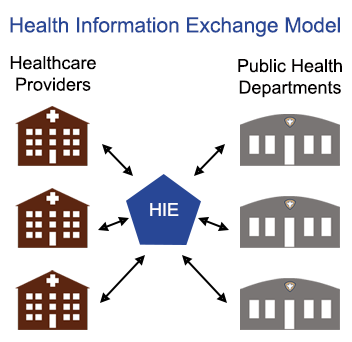
Public Health,
Interoperability
Is the value of participating in Health Information Exchange (HIE) worth it for public health departments?
READ MORE >

Industry News
Dealing with Change: Managing the Uncertainty in Healthcare
READ MORE >

Data Security
8 Common HIPAA Breaches to Avoid and What to Look for in Your EHR
READ MORE >

Practice Management
Check your Tables: Common UDS Reporting Mistakes
READ MORE >

Financial Wellness
Three Steps to Make the Most of EHR Vendor Demos
READ MORE >

Patient Experience,
Expert Interviews
Expert Interview: Marni Mason, BSN, MBA
READ MORE >

Behavioral Health,
Industry News
Addiction in America: A $420 Billion-dollar fight in Behavioral Health
READ MORE >

Medical Billing,
Financial Wellness
Clinicians soon to be rewarded for providing quality patient care with new Medicare Payment System (MACRA)
READ MORE >

Industry News
Affordable Care Act Open Enrollment and Community Health (FQHC)
READ MORE >
Patagonia Health News
Inc. Magazine Ranks Patagonia Health #865 on the 35th Annual List of America’s Fastest-Growing Private Companies—the Inc. 5000
READ MORE >

Patagonia Health News
Reduce Costs and Increase Productivity with Convenient Paperless Integrated Electronic Fax
READ MORE >

Behavioral Health,
Public Health
Without a certified EHR, clinics are missing out on benefits of eRX
READ MORE >

Medical Billing,
Financial Wellness
Accountable Care Organizations and you: What you need to know about ACOs and Community Health
READ MORE >

Financial Wellness
Five Steps to a Successful EHR Data Migration
READ MORE >

Patagonia Health News
Streamline your community health center with improved UDS Reporting options
READ MORE >

Financial Wellness
7 Levels of Service & Support You Should Demand from Your EHR Vendor
READ MORE >

Patagonia Health News
Quick Photo Capture Currently Now in Pilot Mode
READ MORE >

Patagonia Health News
New Bar Code Scanner Option for Immunization App
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Matthew Simon, MA, GISP
READ MORE >

Patagonia Health News
Patagonia Health Expands; Relocates Headquarters
READ MORE >

Behavioral Health,
Public Health
Revised EHR Incentive Program Could Get Behavioral Health and Public Health Agencies on the Same Team
READ MORE >

Industry News,
Financial Wellness
What Federally Qualified Health Centers (FQHC) Need to Know About the 340B Drug Program
READ MORE >

Medical Billing,
Financial Wellness
Are You Down with PPS?
READ MORE >

Financial Wellness
21 Steps to a Successful EHR Implementation
READ MORE >

Practice Management,
Data Security
Understand HIPAA violations to prevent them from happening to you
READ MORE >

Public Health
What's in your Family Planning Annual Report? (FPAR)
READ MORE >

Financial Wellness,
Healthcare Technology
Time is Money: FQHC Requirements from their EHR
READ MORE >

Patagonia Health News
Workflow Analysis & Optimization Services
READ MORE >

Patagonia Health News
Patagonia Health Wins Bronze Stevie® Award in 2016 10th Annual Stevie Awards
READ MORE >

Interoperability,
Expert Interviews
Expert Interview: Ross D. Martin, MD, MHA
READ MORE >

Patagonia Health News
Patagonia Health makes the list of most promising RTP tech companies
READ MORE >

Medical Billing,
Expert Interviews
Expert Interview: Laurie A. Poulin, CPC
READ MORE >

Financial Wellness
Conducting a workflow analysis for public health departments: Will your EHR vendor perform this for you?
READ MORE >

Patagonia Health News
Patagonia Health Carries Customers Safely Across the ICD-10 Threshold
READ MORE >

Public Health
Why Public Health Departments should focus on improving workflow as well as EHR implementation
READ MORE >

Public Health
Credentialing Made Easier for Local Health Departments
READ MORE >

Financial Wellness
EHR User Groups: The Benefits of Group Learning
READ MORE >

Patient Experience,
Patient Portal
The benefits of an EHR from the patient’s perspective
READ MORE >

Industry News,
Practice Management
Implementing EHRs at Title X Agencies
READ MORE >
Patagonia Health News,
Customer Story
Cleveland County Health Department Shares Proven Best Practices for a Successful Public Health EHR Implementation
READ MORE >

Patagonia Health News
Patagonia Health Ranks Top 5% on the Inc. 5000 List Fastest Growing Companies
READ MORE >

Public Health,
Industry News
Patagonia Health EHR; Making things easier for healthcare professionals
READ MORE >
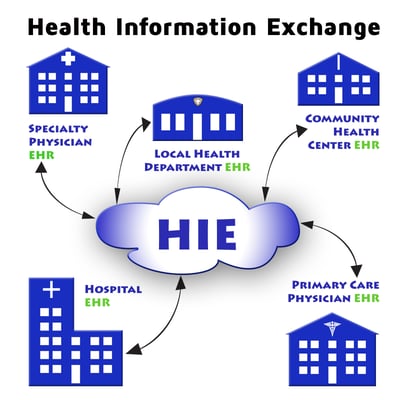
Interoperability
What is Health Information Exchange and Why is it Important for EHR use?
READ MORE >

Practice Management
Now that you have an Electronic Health Record, what is your strategy for scanning medical records?
READ MORE >

Patagonia Health News,
Customer Story
Rowan County Health Department Replaces Legacy EHR Software with Patagonia Health
READ MORE >
Public Health
Working Together: Top 10 Public Health Achievements in 10 year span
READ MORE >

Patagonia Health News,
Customer Story
Patagonia Health Aids County Health Departments to Successful Attestations
READ MORE >

Patagonia Health News
Meaningful Use EHR Incentive Assistance Service
READ MORE >

Data Security
6 Things Your EHR Must Do to Secure Patient Information
READ MORE >

Medical Billing,
Financial Wellness
Three easy steps to increase reimbursements for public health departments
READ MORE >

Patagonia Health News,
Customer Story
Nash County Health Department Switches to Patagonia Health EHR to Improve workflow and Meet Meaningful Use Requirements
READ MORE >

Interoperability,
Practice Management
Connecting to Health Information Exchange (HIE) via EHR - how it helps syndromic surveillance for public health
READ MORE >

Medical Billing
Understanding Medicare Incident-To Billing for Public Health
READ MORE >

Medical Billing,
Public Health
ICD-10-CM has big benefits for Public Health!
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Dr. Stephanie Bailey
READ MORE >

Public Health,
Patient Experience
How can EHR photo IDs help local health departments uniquely identify patients?
READ MORE >

Financial Wellness
The 6 must-haves for an easy to use EHR for Local Health Departments.
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Jan O’Neill
READ MORE >

Public Health,
Interoperability
Timely data is difficult to obtain for urban public health departments
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Kevin Sherin MD, MPH, MBA
READ MORE >
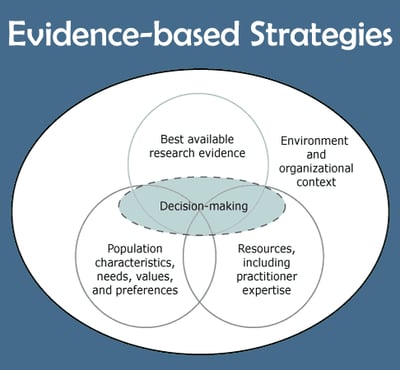
Public Health,
Patient Experience
How can EHR help with Evidence-Based Approach to improving patient health?
READ MORE >
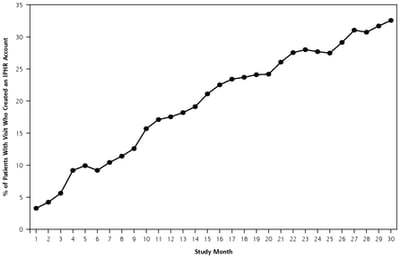
Public Health,
Patient Experience
Are local health departments ready to share medical information via patient portal?
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Anna Schenck
READ MORE >

Patagonia Health News
EHR Dashboard App for local health departments
READ MORE >

Practice Management,
Expert Interviews
Expert Interview: John Graham, PhD
READ MORE >

Public Health
What is Public Health?
READ MORE >

Industry News,
Practice Management
What’s happening with Title X Family Planning?
READ MORE >
Sure, price is important when buying an EHR.
READ MORE >

Public Health,
Patient Experience
Medical Record Errors: They’re more likely than you think.
READ MORE >

Financial Wellness
7+1 bonus steps for EHR selection for local health departments
READ MORE >

Public Health,
Healthcare Technology
The power of data in a blackout: LHD preparedness & emergency response
READ MORE >
Healthcare Technology
When it comes to EHR adoption, where does public health rank?
READ MORE >

Patient Experience,
Industry News
Patients like the convenience of mobile health but doctors are hesitant. Why?
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Dr. Susan Zepeda
READ MORE >

Public Health,
Industry News
Are fist-bumps the new handshake for public health?
READ MORE >
Industry News
The Center for Healthy North Carolina releases its latest “Snapshot of Success”
READ MORE >
Public Health,
Patient Experience
How texting is benefiting public health
READ MORE >

Public Health
Pestronk encourages local health department leaders
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Robert Pestronk
READ MORE >
Public Health
Public health and prevention: From behind the scenes to center stage
READ MORE >
Public Health,
Patient Experience
There’s an app for that: Consider the Advantages of Mobile Technologies for Public Health
READ MORE >

Public Health,
Expert Interviews
Expert Interview: Laura Edwards
READ MORE >
Patagonia Health News
NC HIE Partners with Patagonia Health and NC Office of Rural Health
READ MORE >

Public Health,
Expert Interviews
Expert Interview – Rebecca Williams, MHS, PhD
READ MORE >

Patagonia Health News
Patagonia Health Receives ONC-ACB Certification by Drummond Group
READ MORE >

Patagonia Health News
Public Health Selects Patagonia Health for EHR Billing Solutions
READ MORE >
Patagonia Health News
Patagonia Health launches an EMR offering specially designed for Behavioral Health providers. Behavioral Health specialty treatment forms are built into the EMR tool and are easily accessed, complete
READ MORE >
Patagonia Health News
Patagonia Health EHR is federally certified for Meaningful Use 2011
READ MORE >
Patagonia Health News
Patagonia Health recognized as one of the most promising North Carolina start-ups in 2010
READ MORE >
Load More
Subscribe for More Helpful Content From the Patagonia Health Team!
Need guidance?
Schedule a free 20-minute consultation.
Have questions about how Patagonia Health can meet your needs? Our team of experts is ready to help.